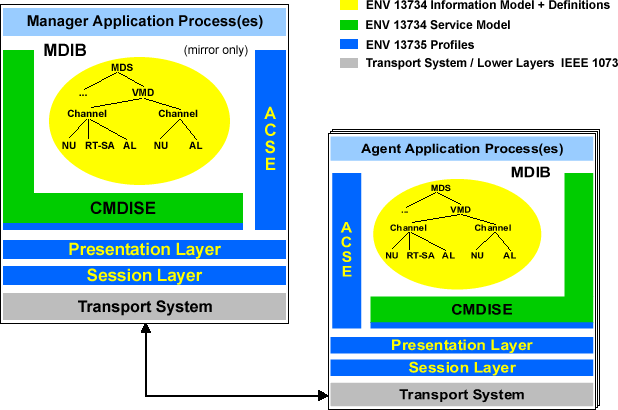
The “VITAL“ communication architecture is based on the ISO management system “agent/manager“-concept. It defines the application layer in the ISO/OSI layer model, thus guaranteeing the flexibility to select the underlying transport protocol. This allows “VITAL“ to be used with Bluetooth, TCP/IP, IrDA or other transmission systems.
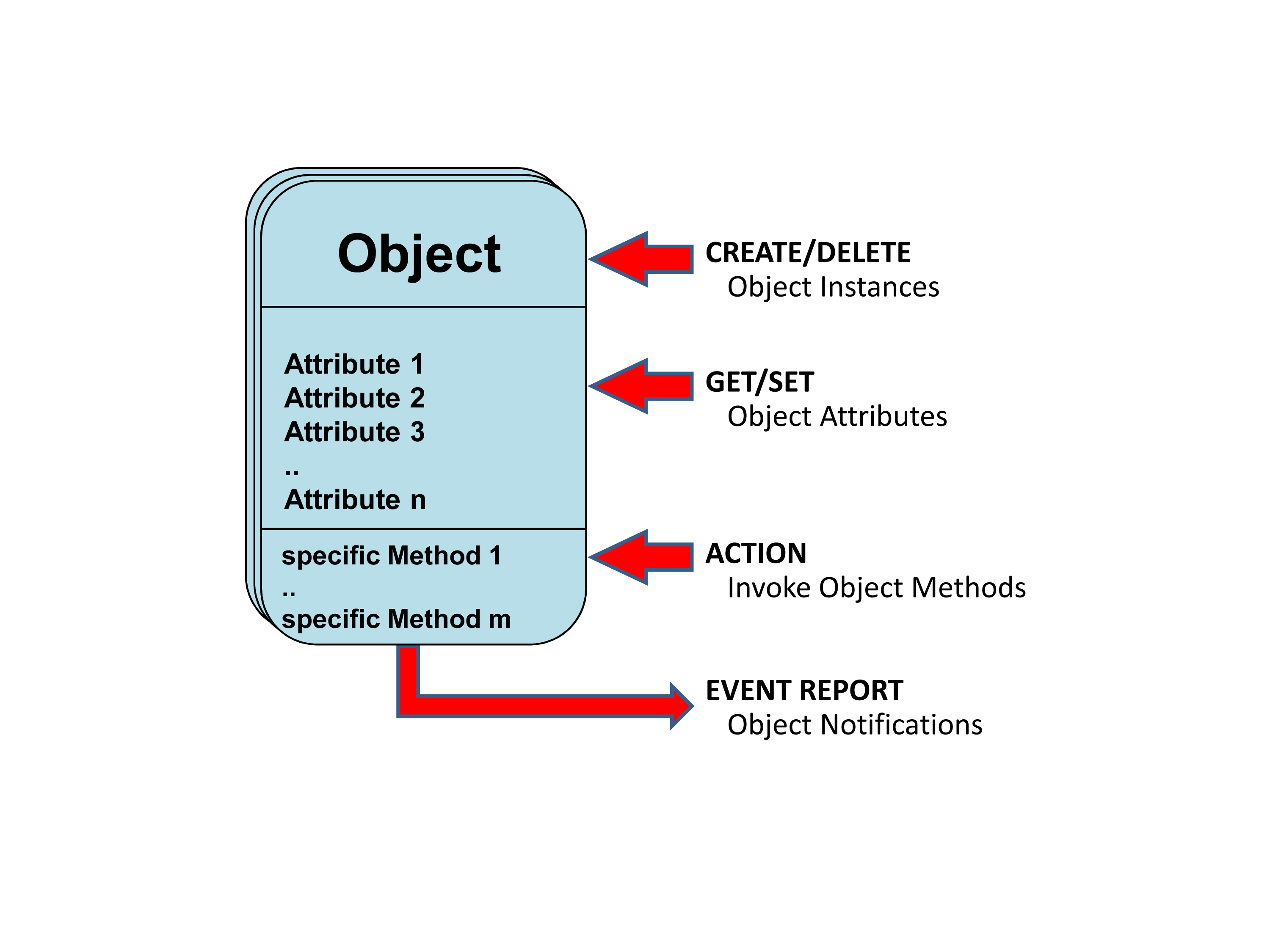
The automated ad hoc equipment communication feature requires that all communicated information elements be clearly implemented in corresponding codes. To do this “VITAL“ relies on an object-oriented information model.
A “medical data information base“ (“MDIB“) on both the agent and manager side of the system contains application-specific object and attribute instances that are defined in the model. In an extensive nomenclature, unique codes were defined for all of the model elements, equipment types, dimensions, measurements, medical measurement values and conditions used in the “MDIB“. In order to clearly denote these codes for developers and users, a special “systematic name“ was introduced that permits distinct semantic correlation across disciplinary and linguistic borders.
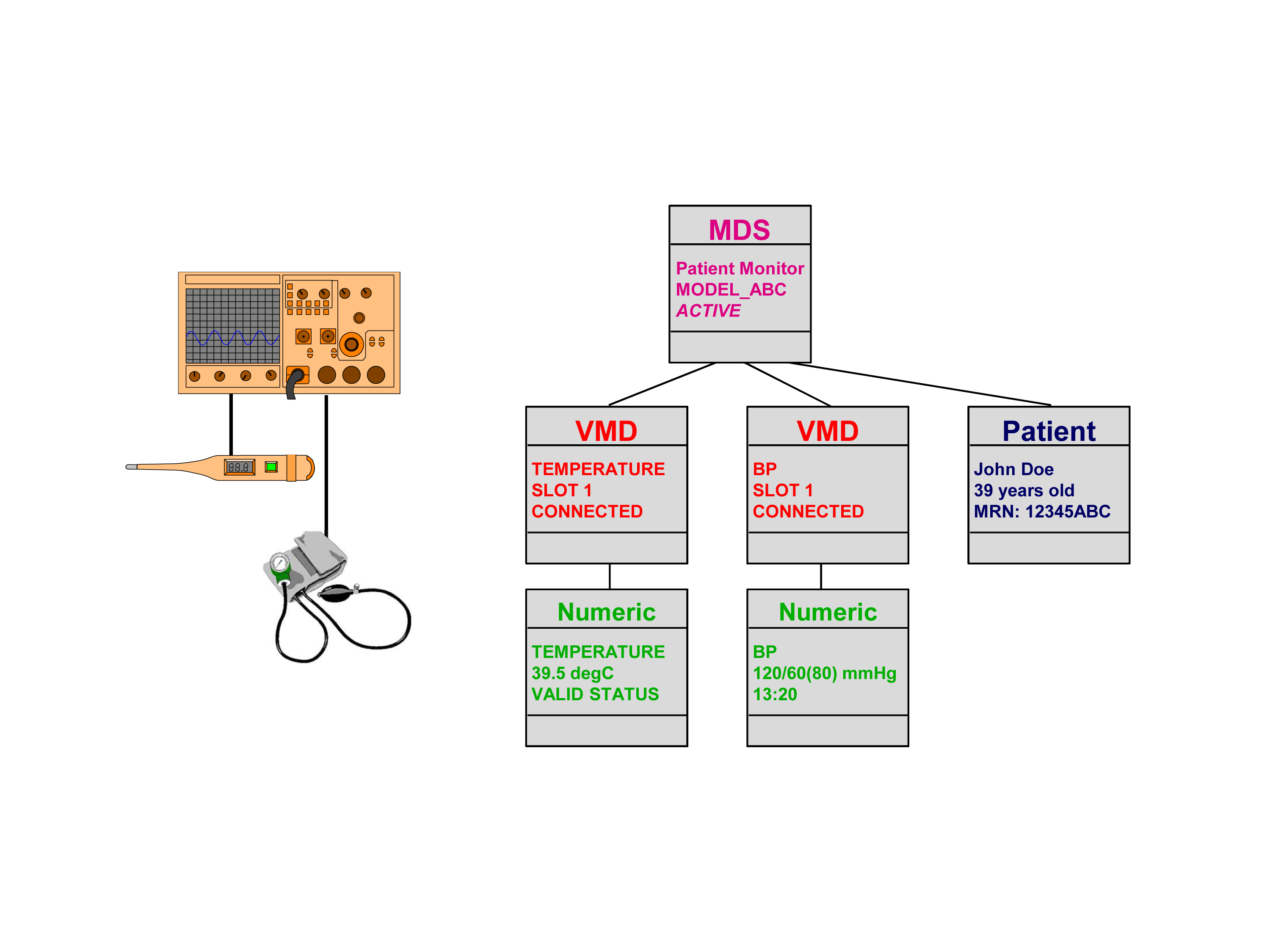
The middle figure depicts an example of the “VITAL“-specific modeling of medical equipment. In this case the model displays thermometer and blood pressure watch values, plus patient-specific data, in a corresponding object hierarchy and then creates an overall system.
Using plug-and-play, any combination of “VITAL“-compatible equipment can be transformed into an interoperable unit. In order to satisfy the wide range of application requirements, from intensive care to home care, the highly-scalable “VITAL“ standard can be implemented with a simple, low-resource “polling mode profile“ or for maximum flexibility with a “base line profile“. “VITAL“ basically establishes the underlying communication behavior, leaving the actual implementation and application design up the programmer. For the information modeling of commonly-used equipment or functions, such as an EKG, corresponding “device profile“ standards help ensure interoperability.