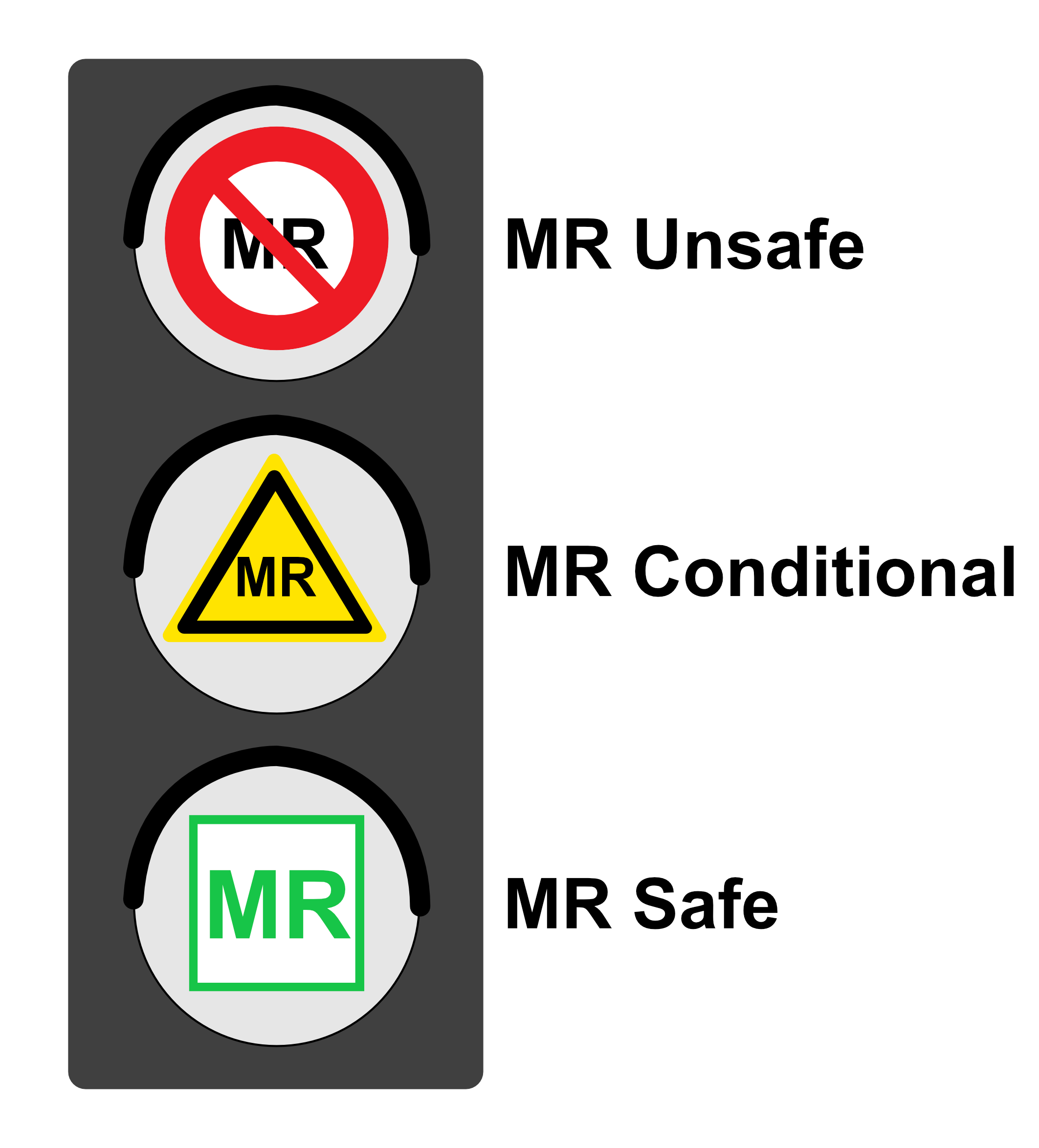
The number of MR examinations performed is constantly increasing. In addition to the development of novel MR devices, implants and procedures, the safety of personnel and patients is a top priority for us.
Through MR compatibility and safety tests on active and passive implants and on MR-relevant devices and accessories, we contribute to the safety of users and patients. Through our expertise in this field, we support research and development work on the next generation of MR-safe medical devices.